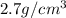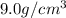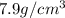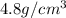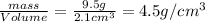Chemistry, 02.07.2019 23:30 nehemiahj85
Marcus measured the masses and volumes of samples of four different substances, and he calculated their densities. the table shows marcus’s measured and calculated values. substance mass (g) volume (cm3) density (g/cm3) aluminum 5.7 2.1 2.7 copper 14.4 1.6 9.0 iron 9.5 1.2 7.9 titanium 8.6 1.8 4.8 next, marcus obtained another sample, which is made of one of the four substances that he had already measured. the table shows marcus’s measured values for this unknown sample. substance mass (g) volume (cm3) ? 9.5 2.1 what is the unknown sample made of?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 23.06.2019 01:30
Which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1

Chemistry, 23.06.2019 03:00
Achemical equilibrium between gaseous reactants and products is shown. n2(g) + 3h2(g) ⇌ 2nh3(g) how will the reaction be affected if the pressure on the system is increased? it will shift toward the reactant side as there is lower pressure on the reactant side. it will shift toward the product side as there is higher pressure on the product side. it will shift toward the reactant side as there are a greater number of moles of gas on the reactant side. it will shift toward the product side as there are a fewer number of moles of gas on the product side.
Answers: 2
You know the right answer?
Marcus measured the masses and volumes of samples of four different substances, and he calculated th...
Questions


Mathematics, 27.11.2020 05:50


Computers and Technology, 27.11.2020 05:50

Geography, 27.11.2020 05:50

Mathematics, 27.11.2020 05:50


Business, 27.11.2020 05:50



Mathematics, 27.11.2020 05:50

Advanced Placement (AP), 27.11.2020 05:50



English, 27.11.2020 05:50



Chemistry, 27.11.2020 05:50


Mathematics, 27.11.2020 05:50