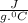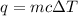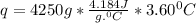Chemistry, 02.07.2019 23:00 fixianstewart
A85.2 g copper bar was heated to 221.32 degrees celsius and placed in a coffee cup calorimeter containing 4250 ml of water at 22.55 degrees celsius. the final temperature of the water was recorded to be 26.15 degrees celsius. how much heat was gained by the water?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:10
There are 6.022 x 10^23 atoms of hg in 1 mole of hg. the number of atoms in 4.5 moles of hg can be found by multiplying 4.5 by 6.022 x 10^23 a. 2.7 x 10^24 b. 27 x 10^23 c. 2.71 x10^24 d. 27.099 x 10^23
Answers: 3

Chemistry, 21.06.2019 20:00
2h2s + 3o2 2so2 + 2h2o which option gives the correct mole ratios? h2s: so2 = 2: 2 and o2: h2o = 3: 2 h2s: so2 = 2: 3 and o2: h2o = 3: 2 h2s: so2 = 4: 4 and o2: h2o = 5: 4 h2s: so2 = 4: 6 and o2: h2o = 4: 4
Answers: 1

Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2
You know the right answer?
A85.2 g copper bar was heated to 221.32 degrees celsius and placed in a coffee cup calorimeter conta...
Questions

Mathematics, 16.09.2019 05:10

English, 16.09.2019 05:10


Mathematics, 16.09.2019 05:10

Mathematics, 16.09.2019 05:10


Biology, 16.09.2019 05:10



Biology, 16.09.2019 05:10


Mathematics, 16.09.2019 05:10


Business, 16.09.2019 05:10

Mathematics, 16.09.2019 05:10


Mathematics, 16.09.2019 05:10



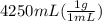 = 4250 g
= 4250 g for water = 26.15 - 22.55 = 3.60 degree C
for water = 26.15 - 22.55 = 3.60 degree C