
Chemistry, 02.07.2019 22:00 ethanm2685
Question 30select the correct answer from each drop-down menu. a reaction proceeds with 2.72 moles of magnesium chlorate and 3.14 moles of sodium hydroxide. this is the equation of the reaction: mg(clo3)2 + 2naoh → mg(oh)2 + 2naclo3. determine the theoretical amount of each product that the reaction will produce. the reaction will produce of magnesium hydroxide and of sodium chlorate. (both have the same dropdown) a. 1.36 b. 1.57 c. 2.72 d. 3.14 e. 4.52 (all in moles)

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 2

Chemistry, 22.06.2019 17:30
Observation and experimentation have led many scientists to accept a theory about the origin of the universe. this theory is called the big bang theory. scientific evidence collected and observed by scientists around the world suggests that the universe is ever expanding from a hot and dense initial state. what makes this a scientific theory? (2 points)
Answers: 2

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3
You know the right answer?
Question 30select the correct answer from each drop-down menu. a reaction proceeds with 2.72 moles o...
Questions

Mathematics, 19.02.2021 08:20


Biology, 19.02.2021 08:20

Chemistry, 19.02.2021 08:20

Mathematics, 19.02.2021 08:20

English, 19.02.2021 08:20

Mathematics, 19.02.2021 08:20

Spanish, 19.02.2021 08:20

Mathematics, 19.02.2021 08:20

Mathematics, 19.02.2021 08:20


Mathematics, 19.02.2021 08:20


Biology, 19.02.2021 08:20

Mathematics, 19.02.2021 08:20


Mathematics, 19.02.2021 08:20

Computers and Technology, 19.02.2021 08:20

Mathematics, 19.02.2021 08:20

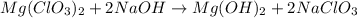
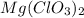 react with 2 mole of
react with 2 mole of 
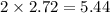 moles of
moles of 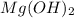 and
and 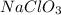 by using the moles of limiting reagent.
by using the moles of limiting reagent.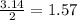 moles of
moles of 

