
The molecule carbon disulfide cs2 is nonpolar and has only london dispersion forces between the molecules. carbon tetrachloride ccl4 is also nonploar but is made larger molecules that are more strongly affected by london dispersion forces. based on this information cs2 likely has a melting point and a vapor pressure when compared with ccl4

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:20
Which type of substance ionizes partially and gives off hydrogen ions when dissolved in water? a. strong acid b. strong base c. weak acid d. weak base
Answers: 1

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 07:00
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1
You know the right answer?
The molecule carbon disulfide cs2 is nonpolar and has only london dispersion forces between the mole...
Questions

Chemistry, 14.09.2020 21:01

English, 14.09.2020 21:01

Mathematics, 14.09.2020 21:01

Biology, 14.09.2020 21:01

Physics, 14.09.2020 21:01

Arts, 14.09.2020 21:01

Mathematics, 14.09.2020 21:01

Physics, 14.09.2020 21:01

English, 14.09.2020 21:01

Mathematics, 14.09.2020 21:01

Chemistry, 14.09.2020 21:01

Mathematics, 14.09.2020 21:01

Chemistry, 14.09.2020 21:01

Mathematics, 14.09.2020 21:01

Chemistry, 14.09.2020 21:01

Chemistry, 14.09.2020 21:01

Chemistry, 14.09.2020 21:01

Mathematics, 14.09.2020 21:01

Mathematics, 14.09.2020 21:01

English, 14.09.2020 21:01

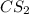 where as the vapor pressure of
where as the vapor pressure of ![CS_2[/tex[ is higher than [tex]CCl_4](/tpl/images/0043/9801/99b5e.png) .
.

