
Chemistry, 02.07.2019 15:30 Jordan0423
How many moles of potassium chloride and oxygen can be produced from 100.0g of potassium chlorate? the balanced equation is: 2 kclo3=2kcl+3o2

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which of the dna typing techniques do you think you would choose if you had to analyze a dna sample? why?
Answers: 1


Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 23.06.2019 04:00
Achemical reaction is done in the setup shown , resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 2
You know the right answer?
How many moles of potassium chloride and oxygen can be produced from 100.0g of potassium chlorate?...
Questions



Mathematics, 08.12.2020 01:00

Chemistry, 08.12.2020 01:00

Social Studies, 08.12.2020 01:00



Chemistry, 08.12.2020 01:00

Social Studies, 08.12.2020 01:00

Business, 08.12.2020 01:00

Mathematics, 08.12.2020 01:00


English, 08.12.2020 01:00

Chemistry, 08.12.2020 01:00


Mathematics, 08.12.2020 01:00


Mathematics, 08.12.2020 01:00


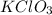 = 100.0 g
= 100.0 g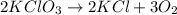
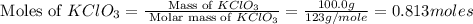
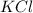 and
and 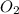 .
.

