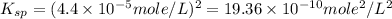Chemistry, 02.07.2019 09:30 jjortiz3137
The solubility of baco3(s) in water at a certain temperature is 4.4 10–5 mol/l. calculate the value of ksp for baco3(s) at this temperature.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1

Chemistry, 22.06.2019 22:30
Why is it possible for different microorganisms to extract energy not only from carbohydrates and other biological molecules but from a large variety of substances?
Answers: 1

Chemistry, 23.06.2019 12:40
Metric temperature is measured in celsius and fahrenheit. true or false
Answers: 2

Chemistry, 23.06.2019 13:20
In the haber reaction, patented by german chemist fritz haber in 1908, dinitrogen gas combines with dihydrogen gas to produce gaseous ammonia. this reaction is now the first step taken to make most of the world's fertilizer. suppose a chemical engineer studying a new catalyst for the haber reaction finds that 671 liters per second of dinitrogen are consumed when the reaction is run at 271c and 0.99atm. calculate the rate at which ammonia is being produced. give your answer in kilograms per second. round your answer to significant digits.
Answers: 3
You know the right answer?
The solubility of baco3(s) in water at a certain temperature is 4.4 10–5 mol/l. calculate the value...
Questions

Mathematics, 26.07.2019 09:30




History, 26.07.2019 09:30

Social Studies, 26.07.2019 09:30

Mathematics, 26.07.2019 09:30




Mathematics, 26.07.2019 09:30



World Languages, 26.07.2019 09:30



History, 26.07.2019 09:30



Health, 26.07.2019 09:30

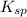 for
for 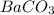 is
is 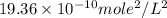 .
.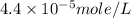
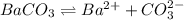
![K_{sp}=[Ba^{2+}][CO^{2-}_3]](/tpl/images/0042/0615/8f6e9.png)
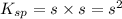
![[Ba^{2+}]=[CO^{2-}_3]=s=4.4\times 10^{-5}mole/L](/tpl/images/0042/0615/c600c.png)