
Type the correct answer in the box. express the answer to three significant figures. given: ch4 + 2o2 → co2 + 2h2o, δh = -890 kj/mol how much energy is released when 59.7 grams of methane (ch4) reacts with oxygen? the combustion of 59.7 grams of methane releases 34.5 kilojoules of energy.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:00
Use examples from the article to explain one positive and one negative effect that chemistry has had on society
Answers: 2


Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

Chemistry, 22.06.2019 18:00
The human activities in two locations are described below: location a: rampant use of plastic containers location b: excessive use of pesticides and fertilizers which statement is most likely true? location a will have poor air quality because plastic is biodegradable. location a will experience water scarcity because plastic absorbs moisture. the population of honeybees will increase in location b because production of crops will increase. the population of fish in location b will decrease because the water is contaminated.
Answers: 1
You know the right answer?
Type the correct answer in the box. express the answer to three significant figures. given: ch4 + 2...
Questions

Biology, 11.10.2020 02:01

Advanced Placement (AP), 11.10.2020 02:01

English, 11.10.2020 02:01

Mathematics, 11.10.2020 02:01


Mathematics, 11.10.2020 02:01

History, 11.10.2020 02:01




Mathematics, 11.10.2020 02:01


Chemistry, 11.10.2020 02:01



Engineering, 11.10.2020 02:01


Physics, 11.10.2020 02:01

Mathematics, 11.10.2020 02:01

Mathematics, 11.10.2020 02:01

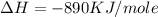
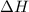 is in negative that means the energy is releasing.
is in negative that means the energy is releasing.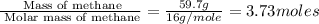
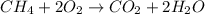
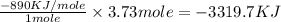 of energy
of energy


