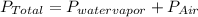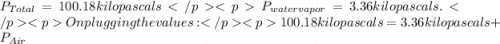Chemistry, 02.07.2019 05:30 heavenwagner
You collect a sample of gases from an indoor pool area. the sample contains air and water vapor. the total pressure is 100.18 kilopascals, and the partial pressure of the water vapor is 3.36 kilopascals. what is the partial pressure of the air in the sample? a. 29.8 kpa b. 51.77 kpa c. 96.82 kpa d. 103.54 kpa e. 337 kpa

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3

Chemistry, 22.06.2019 19:20
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3

Chemistry, 22.06.2019 21:20
The organs inside the body and how they function together
Answers: 3
You know the right answer?
You collect a sample of gases from an indoor pool area. the sample contains air and water vapor. the...
Questions

Mathematics, 21.05.2021 17:40


History, 21.05.2021 17:40

Mathematics, 21.05.2021 17:40




Mathematics, 21.05.2021 17:40

Mathematics, 21.05.2021 17:40




English, 21.05.2021 17:40


English, 21.05.2021 17:40

Mathematics, 21.05.2021 17:40

History, 21.05.2021 17:40

Mathematics, 21.05.2021 17:40