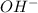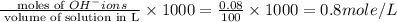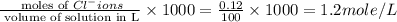Chemistry, 02.07.2019 03:00 stefanylopez731
Calculate the molarity of each type of ion remaining in solution after 20.0 ml of 6.00 m hcl is mixed with 50.0 ml of 2.00 m barium hydroxide and 30.0 ml of water.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 22.06.2019 13:30
Table sugar completely dissolved in water is an example of a?
Answers: 1

Chemistry, 23.06.2019 01:30
If a particle has z = 25 and 23 electrons, what is its charge?
Answers: 2
You know the right answer?
Calculate the molarity of each type of ion remaining in solution after 20.0 ml of 6.00 m hcl is mixe...
Questions



Mathematics, 28.06.2019 11:30


Mathematics, 28.06.2019 11:30



Mathematics, 28.06.2019 11:30

Mathematics, 28.06.2019 11:30

Physics, 28.06.2019 11:30

Mathematics, 28.06.2019 11:30

Mathematics, 28.06.2019 11:30

Biology, 28.06.2019 11:30






English, 28.06.2019 11:30

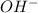 ions = 0.8 mole/L
ions = 0.8 mole/L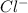 ions = 1.2 mole/L
ions = 1.2 mole/L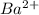 ions = 1 mole/L
ions = 1 mole/L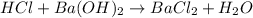
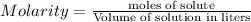
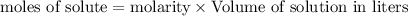
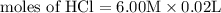 = 0.12
= 0.12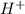 and 1 mole of
and 1 mole of 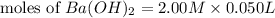 = 0.1
= 0.1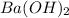 dissociates to give 2 moles of
dissociates to give 2 moles of