
Chemistry, 02.07.2019 03:00 Kenastryker808
What is the ph of a solution with a hydronium (h3o+) concentration of 5.6 × 10-9 m? use the table to you. a. 7.00 b. 8.25 c. 9.00 d. 9.76

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3



Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1
You know the right answer?
What is the ph of a solution with a hydronium (h3o+) concentration of 5.6 × 10-9 m? use the table t...
Questions



Mathematics, 31.03.2020 19:24

English, 31.03.2020 19:24


Mathematics, 31.03.2020 19:24

Physics, 31.03.2020 19:24

Mathematics, 31.03.2020 19:24


Mathematics, 31.03.2020 19:24


Mathematics, 31.03.2020 19:24







Mathematics, 31.03.2020 19:24

History, 31.03.2020 19:24

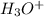 , which is the same as
, which is the same as 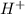 , we use the formula
, we use the formula ![pH= -log [H^{+} ]](/tpl/images/0040/9833/1afcc.png) to find the pH of the solution.
to find the pH of the solution.![pH= -log[H^{+}] = -log [5.6 \times 10^{-9} ] = 8.25](/tpl/images/0040/9833/7f349.png)


