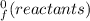Chemistry, 02.07.2019 02:30 hfroslie9840
What is the change in enthalpy for the following reaction? 2h2o2(aq)  2h2o(l) + o2(g) given: h2o: ∆h= -242 kjh2o2: ∆h= -609 kja.-286 kjb. -572 kjc. 572 kjd. 286 kj

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 22.06.2019 17:30
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1

Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2

You know the right answer?
What is the change in enthalpy for the following reaction? 2h2o2(aq)  2h2o(l)&nb...
Questions




English, 18.09.2021 22:10


Mathematics, 18.09.2021 22:10


Mathematics, 18.09.2021 22:10


History, 18.09.2021 22:10

Mathematics, 18.09.2021 22:10


Mathematics, 18.09.2021 22:10

English, 18.09.2021 22:10

Physics, 18.09.2021 22:10


Arts, 18.09.2021 22:10


Mathematics, 18.09.2021 22:10

Advanced Placement (AP), 18.09.2021 22:10

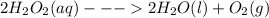
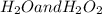 are:
are: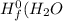 ) = -285.8kJ/mol[/tex]
) = -285.8kJ/mol[/tex]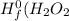 ) = -187.6 kJ/mol[/tex]
) = -187.6 kJ/mol[/tex]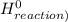 =∑ΔH
=∑ΔH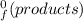 -∑ΔH
-∑ΔH