
Chemistry, 02.07.2019 01:00 pattydixon6
An unbalanced chemical equation is shown: h2o2 → 2h2o + o2 which of the following statements explains why the equation is not balanced? a two h2o2 molecules should decompose to form the given products. b four h2o2 molecules should decompose to form the given products. c two h2o2 molecules should undergo a synthesis reaction to form the given products. d four h2o2 molecules should undergo a synthesis reaction to form the given products.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 22.06.2019 22:00
Imagine one batch of soup (batch “a”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (batch “b”) is made with 8.32 g/can of salt. explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Answers: 2

Chemistry, 22.06.2019 23:10
Amines are good nucleophiles, even though they are neutral molecules. how would the rate of an sn2 reaction between an amine and an alkyl halide be affected if the polarity of the solvent is increased? amines are good nucleophiles, even though they are neutral molecules. how would the rate of an reaction between an amine and an alkyl halide be affected if the polarity of the solvent is increased? because both reactants in the rate-limiting step are neutral, the reaction will be faster if the polarity of the solvent is increased. because both reactants in the rate-limiting step are neutral, the reaction will be slower if the polarity of the solvent is increased. because both reactants in the rate-limiting step are neutral, the reaction will occur at the same rate if the polarity of the solvent is increased. request answer
Answers: 3
You know the right answer?
An unbalanced chemical equation is shown: h2o2 → 2h2o + o2 which of the following statements explai...
Questions



Mathematics, 20.05.2021 19:30


Mathematics, 20.05.2021 19:30

Mathematics, 20.05.2021 19:30

History, 20.05.2021 19:30


Computers and Technology, 20.05.2021 19:30

Mathematics, 20.05.2021 19:30

English, 20.05.2021 19:30



Computers and Technology, 20.05.2021 19:30



Mathematics, 20.05.2021 19:30



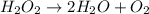
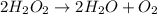
 molecules should decompose to form the given products."
molecules should decompose to form the given products."

