
You discover a chemical reaction that produces 2 moles of valuable propane for every 8 moles of carbon supplied. essentially, this is an exchange rate of 2 moles of propane for every 8 moles of carbon. if you have 1.7 moles of carbon, how many moles of propane can you produce using this reaction?

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 19:30
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2

Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1
You know the right answer?
You discover a chemical reaction that produces 2 moles of valuable propane for every 8 moles of carb...
Questions

Business, 29.09.2019 02:30

Mathematics, 29.09.2019 02:30


Social Studies, 29.09.2019 02:30



Biology, 29.09.2019 02:30


English, 29.09.2019 02:30

History, 29.09.2019 02:30

English, 29.09.2019 02:30


Mathematics, 29.09.2019 02:30

Social Studies, 29.09.2019 02:30


History, 29.09.2019 02:30

English, 29.09.2019 02:30

Chemistry, 29.09.2019 02:30


Mathematics, 29.09.2019 02:30

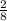 ×1.7 = 0.425 moles
×1.7 = 0.425 moles

