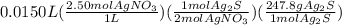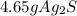Chemistry, 01.07.2019 21:30 jadenpmoore2008
Select the correct answer. if a silver nitrate solution is added to excess sodium sulfide, this reaction takes place: 2agno3(aq) + na2s(aq) → ag2s(s) + 2nano3(aq). suppose you use 0.0150 liter of a 2.50 m solution of silver nitrate. assuming the reaction goes to completion, how much silver sulfide is produced? use the periodic table. a. 1.49 g b. 4.65 g c. 9.30 g d. 18.6 g

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3

Chemistry, 22.06.2019 10:00
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1

Chemistry, 22.06.2019 22:10
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1

Chemistry, 23.06.2019 00:30
An unknown insoluble substance displaced the water shown. it's mass is indicated on the triple beam balance. mass = a. 694 b. 693.5 c. 693.0 d.693.8
Answers: 1
You know the right answer?
Select the correct answer. if a silver nitrate solution is added to excess sodium sulfide, this reac...
Questions

Computers and Technology, 19.07.2019 08:00

History, 19.07.2019 08:00

History, 19.07.2019 08:00

Biology, 19.07.2019 08:00

Computers and Technology, 19.07.2019 08:00

Biology, 19.07.2019 08:00







Computers and Technology, 19.07.2019 08:00

Health, 19.07.2019 08:00

Computers and Technology, 19.07.2019 08:00

Mathematics, 19.07.2019 08:00


Mathematics, 19.07.2019 08:00


History, 19.07.2019 08:00

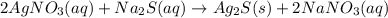
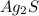 = 2(107.87)+32.06 = 247.8 g per mol
= 2(107.87)+32.06 = 247.8 g per mol