
Chemistry, 01.07.2019 19:00 gus2006santos
What volume of h2o is formed at stp when 6.0g of al is treated with excess naoh? naoh + al + h2o —-> naal(oh)4 + h2 (g)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3

Chemistry, 22.06.2019 08:00
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3
You know the right answer?
What volume of h2o is formed at stp when 6.0g of al is treated with excess naoh? naoh + al + h2o —-...
Questions

Mathematics, 15.01.2021 19:00

Biology, 15.01.2021 19:00

Mathematics, 15.01.2021 19:00


Mathematics, 15.01.2021 19:00





Mathematics, 15.01.2021 19:00





Biology, 15.01.2021 19:10




English, 15.01.2021 19:10

Mathematics, 15.01.2021 19:10

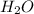 is 14.784 L.
is 14.784 L.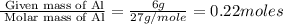
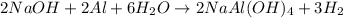
 of
of 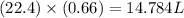 volume of
volume of 

