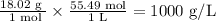Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1

Chemistry, 23.06.2019 01:30
Some molecular compounds, such as hcl, ionize completely in solution. for molecular compounds such as h2co3, most molecules do not ionize in solution. which describes the properties of these two solutes? a. hcl and h2co3 have the same effect on the properties of the solution. b. hcl raises the freezing point of water more than h2co3 does. c. hcl raises the boiling point of water more than h2co3 does.
Answers: 2

Chemistry, 23.06.2019 04:31
What is the amount of energy for a photon that has a 125 cm wavelength
Answers: 2

Chemistry, 23.06.2019 07:00
The image compares the arrangement of electrons in two different neutral atoms. a figure labeled atom q has a shaded sphere at the center of three concentric circles. the innermost circle has two black spheres. the middle circle has six black spheres. to the left of this figure is another figure labeled atom p. atom p has a shaded sphere at the center of three concentric circles. the innermost circle has two black spheres. the middle circle has seven black spheres. which of the following best explains the position of the two atoms in the periodic table?
Answers: 2
You know the right answer?
Which conversion factor would be used to convert g/mol to g/l? need 20 points! a l/mol b mol/g c...
Questions


Social Studies, 26.10.2020 22:50

Mathematics, 26.10.2020 22:50

Computers and Technology, 26.10.2020 22:50





History, 26.10.2020 22:50

Mathematics, 26.10.2020 22:50

Mathematics, 26.10.2020 22:50

Spanish, 26.10.2020 22:50


Advanced Placement (AP), 26.10.2020 22:50




History, 26.10.2020 22:50


Advanced Placement (AP), 26.10.2020 22:50