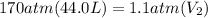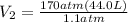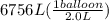Chemistry, 01.07.2019 17:30 2020davidhines
Acylinder containing 44.0 l of helium gas at a pressure of 170 atm is to be used to fill toy balloons to a pressure of 1.1 atm. each inflated balloon has a volume of 2.0 l. what is the maximum number of balloons that can be inflated? (remember that 44.0 l of helium at 1.1 atm pressure will remain in the 'exhausted' cylinder) round your answer to two significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
If you burn 10 kilograms of wood in a fire (combustion) what is the weight of the products after the fire has finished burning the wood?
Answers: 3

Chemistry, 22.06.2019 15:00
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2

Chemistry, 23.06.2019 07:00
In order for a high temperature boiler or steam engine to produce superheated water, or steam: the heat source must be greater than 100°c the water must be permitted to evaporate quickly the system must be sealed and become pressurized above atmospheric pressure the vapor pressure must be kept below 760 mm(hg)
Answers: 1

Chemistry, 23.06.2019 07:00
Scuba divers use tanks of compressed air to them breathe. gases can be compressed because?
Answers: 1
You know the right answer?
Acylinder containing 44.0 l of helium gas at a pressure of 170 atm is to be used to fill toy balloon...
Questions


Mathematics, 22.02.2020 08:50

Mathematics, 22.02.2020 08:57


History, 22.02.2020 08:57


Mathematics, 22.02.2020 08:58


Biology, 22.02.2020 08:58



Biology, 22.02.2020 08:59


Mathematics, 22.02.2020 09:00



Social Studies, 22.02.2020 09:01



Mathematics, 22.02.2020 09:01

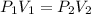
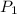 = 170 atm
= 170 atm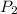 = 1.1 atm
= 1.1 atm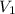 = 44.0 L
= 44.0 L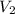 = ?
= ?