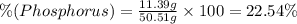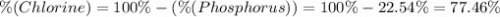Chemistry, 01.07.2019 17:00 hobbs4ever1
A50.51g sample of a compound made from phosphorus and chlorine is decomposed. analysis of the products showed that 11.39 g of phosphorus atoms were produced. answer using three significant figures. what is the percent by mass of phosphorus? % what is the percent by mass of chlorine? %

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the maximum amount of al2(so4)3 which could be formed from 15.84 g of al and 12.89 g of cuso4?
Answers: 2

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3

Chemistry, 23.06.2019 01:00
Aman applies a force of 500n to push a truck 100m down the street how much does he do?
Answers: 1

Chemistry, 23.06.2019 03:00
Abaker touches a pie right after taking it out of the oven. which statement best explains why the pie feels hot?
Answers: 1
You know the right answer?
A50.51g sample of a compound made from phosphorus and chlorine is decomposed. analysis of the produc...
Questions

Mathematics, 08.07.2019 17:30

English, 08.07.2019 17:30



Mathematics, 08.07.2019 17:30



Mathematics, 08.07.2019 17:30


History, 08.07.2019 17:30

Biology, 08.07.2019 17:30

Biology, 08.07.2019 17:30

Biology, 08.07.2019 17:30


Social Studies, 08.07.2019 17:30



Mathematics, 08.07.2019 17:30


History, 08.07.2019 17:30