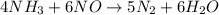For the reaction nh3 + no → n2 + h2o, identify the reactants, products, and their coefficients once the equation is balanced. reactants: 2nh3 and 2no; products: 2n2 and 3h2o products: 2nh3 and 3no; reactants: 2n2 and 3h2o reactants: 4nh3 and 6no; products: 5n2 and 6h2o products: 4nh3 and 2no; reactants: 3n2 and 6h2o

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Electrons are extremely important to what area of technology? a) anti-aging research b) household product development c) electronics d) drug discovery
Answers: 3


Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1

Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2
You know the right answer?
For the reaction nh3 + no → n2 + h2o, identify the reactants, products, and their coefficients once...
Questions


Social Studies, 08.12.2021 15:00

English, 08.12.2021 15:00

English, 08.12.2021 15:10

Engineering, 08.12.2021 15:10


Computers and Technology, 08.12.2021 15:10

English, 08.12.2021 15:10

Mathematics, 08.12.2021 15:10


Mathematics, 08.12.2021 15:10

Business, 08.12.2021 15:10



Biology, 08.12.2021 15:10

Advanced Placement (AP), 08.12.2021 15:10



Mathematics, 08.12.2021 15:10

SAT, 08.12.2021 15:10

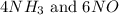
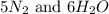
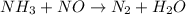
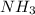 , the coefficient '6' put before the
, the coefficient '6' put before the 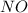 , the coefficient '5' put before the
, the coefficient '5' put before the 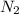 and the coefficient '6' put before the
and the coefficient '6' put before the 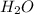 and then we get the balanced chemical equation.
and then we get the balanced chemical equation.