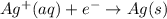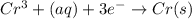Chemistry, 01.07.2019 06:30 trosclairozlynn02
The following equations are half reactions and reduction potentials. ag+ (aq) + e– ag(s) has a reduction potential of +0.80 v. cr3+ (aq) + 3e– cr(s) has a reduction potential of –0.74 v. which statement best compares the substances in these half reactions? a silver ion gains electrons more easily and is a stronger oxidizing agent than a chromium(iii) ion. a silver ion loses electrons more easily and is a stronger oxidizing agent than a chromium(iii) ion. a silver ion gains electrons more easily and is a stronger reducing agent than a chromium(iii) ion. a silver ion loses electrons more easily and is a stronger reducing agent than a chromium(iii) ion. the answer is a

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 18:30
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1

You know the right answer?
The following equations are half reactions and reduction potentials. ag+ (aq) + e– ag(s) has a reduc...
Questions


Mathematics, 01.03.2021 23:30

Biology, 01.03.2021 23:30




Mathematics, 01.03.2021 23:30


Mathematics, 01.03.2021 23:30

Chemistry, 01.03.2021 23:30

Computers and Technology, 01.03.2021 23:30

Advanced Placement (AP), 01.03.2021 23:30

Mathematics, 01.03.2021 23:30

Biology, 01.03.2021 23:30

Mathematics, 01.03.2021 23:30

English, 01.03.2021 23:30




Chemistry, 01.03.2021 23:30