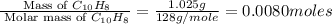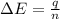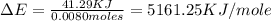Chemistry, 01.07.2019 03:00 Jadamachado45
When 1.025 g of naphthalene (c10h8) burns in a bomb calorimeter, the temperature rises from 24.25°c to 32.33°c. find δerxn for the combustion of naphthalene. the heat capacity of the calorimeter is 5.11 kj/°c?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
The overall chemical reaction for photosynthesis isshown below: 6co2 + 6h20 → c6h12o6 + 602what mass of glucose (c6h1206) can form from71.89 g co2? (molar mass of c6h1206 = 180.18g/mol; molar mass of co2 = 44.01 g/mol)71.89 g co2=g c6h1206
Answers: 1

Chemistry, 22.06.2019 07:00
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2

Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3
You know the right answer?
When 1.025 g of naphthalene (c10h8) burns in a bomb calorimeter, the temperature rises from 24.25°c...
Questions

French, 13.11.2020 23:50

English, 13.11.2020 23:50



History, 13.11.2020 23:50

English, 13.11.2020 23:50

Mathematics, 13.11.2020 23:50



Mathematics, 13.11.2020 23:50


Mathematics, 13.11.2020 23:50

Mathematics, 13.11.2020 23:50


Chemistry, 13.11.2020 23:50

Social Studies, 13.11.2020 23:50



Mathematics, 13.11.2020 23:50

English, 13.11.2020 23:50

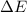 for the combustion of naphthalene is
for the combustion of naphthalene is 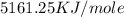
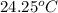
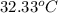
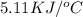
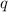
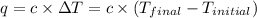
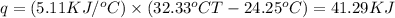
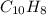 =
=