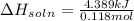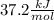Chemistry, 01.07.2019 03:00 paigeisawesome
Student conducts an experiment to determine the enthalpy of solution for lithium chloride dissolved in water. the student combines 5.00 grams of lithium chloride with 100.0 ml of distilled water. the initial temperature of the water is 23.0â°c and the highest temperature after mixing reaches 33.0â°c. assume a density of 1.00 g/ml and a specific heat of 4.18 .

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2


Chemistry, 22.06.2019 11:20
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2

Chemistry, 22.06.2019 14:30
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1
You know the right answer?
Student conducts an experiment to determine the enthalpy of solution for lithium chloride dissolved...
Questions

Biology, 08.12.2021 20:20

Physics, 08.12.2021 20:20


History, 08.12.2021 20:20


Social Studies, 08.12.2021 20:20


Geography, 08.12.2021 20:20

Mathematics, 08.12.2021 20:20


Mathematics, 08.12.2021 20:20




Mathematics, 08.12.2021 20:20

Social Studies, 08.12.2021 20:20


Mathematics, 08.12.2021 20:20

Mathematics, 08.12.2021 20:20

Mathematics, 08.12.2021 20:20

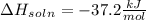
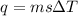
 = change in temperature
= change in temperature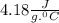
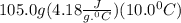
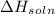 we convert q to kJ and divide by the moles of solute.
we convert q to kJ and divide by the moles of solute.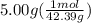
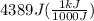 = 4.389 kJ
= 4.389 kJ