
Chemistry, 01.07.2019 01:30 iiwolfiexuni
The hydrogen atom is not actually electronegative enough to form bonds to xenon. were the xenon-hydrogen bond to exist, what would be the structure of xeh4? double-click any atom and type xe to change the label. draw the molecule by placing atoms on the grid and connecting them with bonds. show all lone pairs of electrons.

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2
You know the right answer?
The hydrogen atom is not actually electronegative enough to form bonds to xenon. were the xenon-hydr...
Questions


English, 28.01.2020 18:06

History, 28.01.2020 18:06



English, 28.01.2020 18:06


Mathematics, 28.01.2020 18:06



Mathematics, 28.01.2020 18:06

Mathematics, 28.01.2020 18:06

Social Studies, 28.01.2020 18:06


Mathematics, 28.01.2020 18:06

Social Studies, 28.01.2020 18:06




Geography, 28.01.2020 18:06

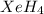 will be square-planar.
will be square-planar.

