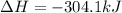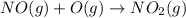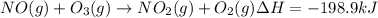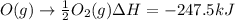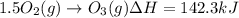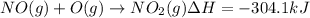Chemistry, 01.07.2019 00:30 jerseygirl1783
Calculate the enthalpy change for the reaction no(g) + o(g) → no2(g) from the following data: no(g) + o3(g) → no2(g) + o2(g) δh = –198.9 kj o3(g) → 1.5o2(g) δh = –142.3 kj o2(g) → 2o(g) δh = 495.0 kj

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Explain which group an element with the electron configuration 1s2 2s2 2p6 3s2 3p6 3d1 4s2 belongs to.
Answers: 3

Chemistry, 22.06.2019 05:20
Why does the sun appear to be the brightest star in the sky? a- its apparent brightness is much greater than other stars. b- it burns more gas, making it brighter than any other star. c- it is the largest star in the galaxy, so it is the brightest star. d- its relative distance to earth is closer than the other stars.
Answers: 1


Chemistry, 22.06.2019 16:00
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1
You know the right answer?
Calculate the enthalpy change for the reaction no(g) + o(g) → no2(g) from the following data: no(g)...
Questions

Computers and Technology, 01.12.2021 01:00



Physics, 01.12.2021 01:00

English, 01.12.2021 01:00

Computers and Technology, 01.12.2021 01:00






Chemistry, 01.12.2021 01:00

English, 01.12.2021 01:00




English, 01.12.2021 01:00

Mathematics, 01.12.2021 01:00