Chemistry, 01.07.2019 00:00 lalala1212
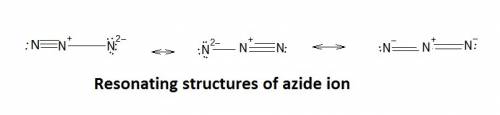
The azide ion, n−3, is a symmetrical ion, all of whose contributing structures have formal charges. draw three important contributing structures for this ion. draw the molecule by placing atoms on the grid and connecting them with bonds. include all nonbonding electrons. show the formal charges of all atoms.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Which statement justifies that phosphine (ph3) is a polar molecule?
Answers: 1


Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2

Chemistry, 22.06.2019 17:30
Aroller coaster is traveling at 13 mi./s when you purchase a hill that is 400 m long and down the hill exonerate at 4.0 m/s squared what is the final velocity of the posterior found your answer to the nearest number
Answers: 1
You know the right answer?
The azide ion, n−3, is a symmetrical ion, all of whose contributing structures have formal charges....
Questions

Mathematics, 16.03.2020 21:33

Mathematics, 16.03.2020 21:34

Mathematics, 16.03.2020 21:34


Mathematics, 16.03.2020 21:34

Mathematics, 16.03.2020 21:34


Biology, 16.03.2020 21:34

English, 16.03.2020 21:34


Health, 16.03.2020 21:34

Biology, 16.03.2020 21:34

History, 16.03.2020 21:34

Biology, 16.03.2020 21:34



Computers and Technology, 16.03.2020 21:34



History, 16.03.2020 21:34

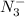 , is a symmetrical ion, all of whose contributing structures have formal charges.
, is a symmetrical ion, all of whose contributing structures have formal charges.