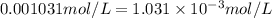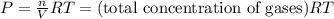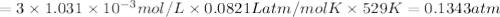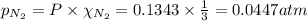Chemistry, 30.06.2019 21:30 nathaliapachon1254
For the reaction n2(g) + 3 h2(g) = 2 nh3(g), kp = 0.0489 at 256˚c. for parts a through d, assume that the reaction is at this temperature. c) a third equilibrium mixture contains 0.0100 mol/l of n2 and 0.0300 mol/l of nh3.what is the concentration of h2 in this mixture? d) a fourth equilibrium mixture contains equal concentrations of all three chemicals. what is the pressure of each substance in this mixture? me on c & d by 11/25 11pm !


Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:40
If equal masses of the listed metals were collected , which would have a greatest volume ? a. aluminum 2.70,b.zinc7.14,c.copper 8.92,d.lead 11.34
Answers: 2

Chemistry, 22.06.2019 08:30
The mass of a neutron is equal to the mass of a proton plus the mass of an electron. true or false false true
Answers: 1

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 22.06.2019 20:00
State one important difference between a physical change and a chemical change?
Answers: 1
You know the right answer?
For the reaction n2(g) + 3 h2(g) = 2 nh3(g), kp = 0.0489 at 256˚c. for parts a through d, assume tha...
Questions

Business, 25.05.2021 07:20

Mathematics, 25.05.2021 07:20

Mathematics, 25.05.2021 07:20

Chemistry, 25.05.2021 07:20

French, 25.05.2021 07:20

English, 25.05.2021 07:20

Mathematics, 25.05.2021 07:20

History, 25.05.2021 07:20

Mathematics, 25.05.2021 07:20


Arts, 25.05.2021 07:20

Mathematics, 25.05.2021 07:20


Mathematics, 25.05.2021 07:20


Mathematics, 25.05.2021 07:20


History, 25.05.2021 07:20


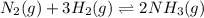
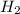 in this mixture
in this mixture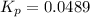
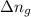 :
: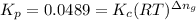
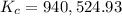
![K_c=940,524.93=\frac{[NH_3]^2}{[N_2][H_2]^3}=\frac{(0.0300 mol/L)^3}{0.0100 mol/L\times [H_2]^3}](/tpl/images/0036/3201/0fc67.png)
![[H_2]^3=10,450,277](/tpl/images/0036/3201/9b237.png)
![[H_2]=218.62 mol/L](/tpl/images/0036/3201/d8502.png)
![[N_2]=[H_2]=[NH_3]=x mol/L](/tpl/images/0036/3201/a39fc.png)
![K_c=940,524.93=\frac{[NH_3]^2}{[N_2][H_2]^3}=\frac{x^2}{x\times x^3}=\frac{1}{x^2}](/tpl/images/0036/3201/ddb76.png)