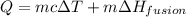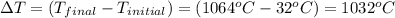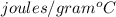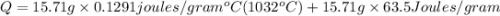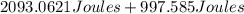The temperature of 15.71 grams of gold rises from 32°c to 1,064°c, and then the gold melts completely. if gold’s specific heat is 0.1291 joules/gram degree celsius and its heat of fusion is 63.5 joules/gram, how much energy is gained by the gold? the gold gained a total of joules of energy.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
If a bottle of olive oil contains 1.4 kg of olive oil, what is the volume, in milliliters ( ml ), of the olive oil?
Answers: 1

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1


Chemistry, 22.06.2019 06:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1
You know the right answer?
The temperature of 15.71 grams of gold rises from 32°c to 1,064°c, and then the gold melts completel...
Questions


Mathematics, 11.02.2021 01:20


Chemistry, 11.02.2021 01:20

Mathematics, 11.02.2021 01:20

Mathematics, 11.02.2021 01:20

Advanced Placement (AP), 11.02.2021 01:20


Mathematics, 11.02.2021 01:20

Arts, 11.02.2021 01:20


English, 11.02.2021 01:20


Mathematics, 11.02.2021 01:20

Mathematics, 11.02.2021 01:20

Mathematics, 11.02.2021 01:20

Computers and Technology, 11.02.2021 01:20