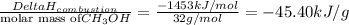Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:00
When light strikes a plane mirror, images form in locations where light does not actually reach. it only appears to the observer as though the light were coming from this position. what type of image is formed?
Answers: 2


Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1
You know the right answer?
Methanol (ch3oh) has been proposed as an alternative fuel. calculate the standard enthalpy of combus...
Questions

English, 12.11.2020 21:00



Computers and Technology, 12.11.2020 21:00

Mathematics, 12.11.2020 21:00


Mathematics, 12.11.2020 21:00

Mathematics, 12.11.2020 21:00






Mathematics, 12.11.2020 21:00

Business, 12.11.2020 21:00


Mathematics, 12.11.2020 21:00

Chemistry, 12.11.2020 21:00


Mathematics, 12.11.2020 21:00

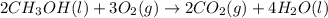
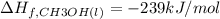

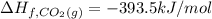
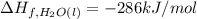
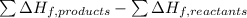
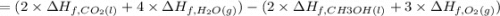
![=[2\times (-393.5 kJ/mol)+4\times (-286 kJ/mol)]-[2\times (-239 kJ/mol)+3\times (0 kJ/mol)]=-1453 kj/mol](/tpl/images/0035/4633/40853.png)