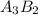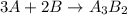Chemistry, 30.06.2019 14:30 sashajayne8260
You are balancing an ionic compound. let's call it: ab. ion a has a charge of +2 and ion b has a charge of -3. therefore, you determine that the ionic compound (ab) will have a ratio of 3 as for every 2 bs. how would you modify ab in order to show that they have this 3-to-2 ratio? question 2 options: 3a2b a3b2 a3b2

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 10:30
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1

Chemistry, 22.06.2019 14:00
The two naturally occurring isotopes of chlorine are 35cl (34.969 amu, 75.77%) and 37cl (36.966 amu, 24.23%). the two naturally occurring isotopes of bromine are 79br (78.918 rm amu, 50.69%) and 81br (80.916 amu, 49.31%). chlorine and bromine combine to form bromine monochloride, brcl. 1. how many peaks will be present in a mass spectrum for brcl? the four combinations of molecule possible given these four isotopes are: 81br37cl, 81br35cl, 79br37cl, and 79br35cl. 2. what are the masses of the four different brcl molecules? express the masses using six significant figures, in decreasing numeric order (highest to lowest), separated by commas.
Answers: 3
You know the right answer?
You are balancing an ionic compound. let's call it: ab. ion a has a charge of +2 and ion b has a c...
Questions

Mathematics, 12.04.2020 01:32



Spanish, 12.04.2020 01:32






Physics, 12.04.2020 01:32


Mathematics, 12.04.2020 01:32





Mathematics, 12.04.2020 01:32

Chemistry, 12.04.2020 01:32


History, 12.04.2020 01:32