Chemistry, 30.06.2019 12:00 JuniperGalaxy
Sucrose (c12h22o11, table sugar) is oxidized in the body by o2 via a complex set of reactions that ultimately produces co2(g) and h2o(l) and releases 5.16 × 103 kj of heat per mole of sucrose. (a) write a balanced thermochemical equation for this reaction. include the physical state of each reactant and product. → enter your answer for δhrxn in scientific notation. δhrxn

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:30
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2

Chemistry, 22.06.2019 23:40
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2

Chemistry, 23.06.2019 00:30
Arrange the elements in order of increasing electronegativity. use the periodic table to you arrange the elements. p o k mg
Answers: 2

Chemistry, 23.06.2019 01:30
Select the correct answer from each drop-down menu. to make a table of the elements, dmitri mendeleev sorted the elements according to their . he then split the list of elements into several columns so that elements beside each other had similar .
Answers: 2
You know the right answer?
Sucrose (c12h22o11, table sugar) is oxidized in the body by o2 via a complex set of reactions that u...
Questions


Mathematics, 28.09.2019 03:00

History, 28.09.2019 03:00

History, 28.09.2019 03:00

Health, 28.09.2019 03:00


Mathematics, 28.09.2019 03:00

Mathematics, 28.09.2019 03:00

English, 28.09.2019 03:00



Spanish, 28.09.2019 03:00




Mathematics, 28.09.2019 03:00

Chemistry, 28.09.2019 03:00




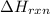 is represented in scientific notation as,
is represented in scientific notation as,