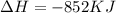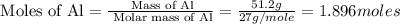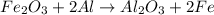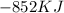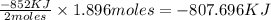Chemistry, 30.06.2019 11:30 nonjabulomabaso6850
How much energy is evolved during the reaction of 51.2 g of al, according to the reaction below? assume that there is excess fe2o3. fe2o3(s) + 2 al(s) â al2o3(s) + 2 fe(s) î´hâ°rxn = -852 kj?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:20
An aqueous solution of calcium hydroxide is standardized by titration with a 0.120 m solution of hydrobromic acid. if 16.5 ml of base are required to neutralize 27.5 ml of the acid, what is the molarity of the calcium hydroxide solution?
Answers: 3

Chemistry, 21.06.2019 22:30
If you want to create an electrical current, which situation would produce a solution capable of this
Answers: 3

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

Chemistry, 23.06.2019 02:50
Select the correct location on the image identify the element that humans need to breathe. 2015 er r ights reserved
Answers: 3
You know the right answer?
How much energy is evolved during the reaction of 51.2 g of al, according to the reaction below? as...
Questions

History, 10.11.2020 21:30





Social Studies, 10.11.2020 21:30

Spanish, 10.11.2020 21:30

Mathematics, 10.11.2020 21:30


Biology, 10.11.2020 21:30


Geography, 10.11.2020 21:30

Biology, 10.11.2020 21:30


Mathematics, 10.11.2020 21:30

Mathematics, 10.11.2020 21:30


Physics, 10.11.2020 21:30


Computers and Technology, 10.11.2020 21:30

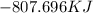 .
.