
State whether each of these statements is true or false. (a) a carbon–carbon triple bond is shorter than a carbon–carbon single bond. (b) there are exactly six bonding electrons in the o2 molecule. (c) the c—o bond in carbon monoxide is longer than the c—o bond in carbon dioxide. (d) the o—o bond in ozone is shorter than the o—o bond in o2. (e) the more electronegative the atom, the more bonds it makes to other atoms.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3

Chemistry, 22.06.2019 19:00
Structure of the atoms: discovery of the nucleus in 1909i need answering all of these questions
Answers: 3

Chemistry, 22.06.2019 23:30
If maltose undergoes hydrolysis what subunits does it results to?
Answers: 2

Chemistry, 23.06.2019 04:20
The reaction below shows a system in equilibrium. how would a decrease in temperature affect this reaction? a. the rate of formation of the gases would increase. b. the equilibrium of the reaction would shift to the left. c. the equilibrium would shift to cause the gases to sublime into solids. d. the chemicals on the left would quickly form the chemical on the right.
Answers: 1
You know the right answer?
State whether each of these statements is true or false. (a) a carbon–carbon triple bond is shorter...
Questions




Mathematics, 28.09.2019 04:30

Mathematics, 28.09.2019 04:30

Mathematics, 28.09.2019 04:30

History, 28.09.2019 04:30


Biology, 28.09.2019 04:30




Mathematics, 28.09.2019 04:30

Health, 28.09.2019 04:30

History, 28.09.2019 04:30



History, 28.09.2019 04:30

Spanish, 28.09.2019 04:30

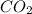 the bond order of (C-O) bond is 2. Higher the bond order shorter will be the bond length.
the bond order of (C-O) bond is 2. Higher the bond order shorter will be the bond length.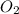 the bond order of (O-O) bond is 2. Higher the bond order shorter will be the bond length.
the bond order of (O-O) bond is 2. Higher the bond order shorter will be the bond length.

