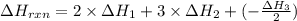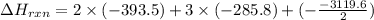Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
If 34.2 grams of lithium react with excess water, how many liters of hydrogen gas can be produced at 299 kelvin and 1.21 atmospheres? 2 li (s) + 2 h2o (l) yields 2 lioh (aq) + h2 (g)
Answers: 3

Chemistry, 22.06.2019 04:30
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1
You know the right answer?
C(graphite) + o2(g) → co2(g)δh o rxn = −393.5 kj/mol h2(g) + 1 2 o2(g) → h2o(l)δh o rxn = −285.8 kj/...
Questions

Mathematics, 14.04.2021 02:20








Mathematics, 14.04.2021 02:20

Geography, 14.04.2021 02:20





Mathematics, 14.04.2021 02:20




English, 14.04.2021 02:20


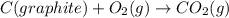
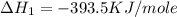
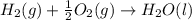
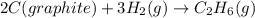
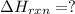
![2\times eq.(1)+\frac{1}{2}(eq.3)\text{[reversing equation 3 and dividing it by 2]}+3(eq.2)](/tpl/images/0034/7108/524c9.png)