
Chemistry, 30.06.2019 11:00 Imamdiallo18
In the balanced equation , cs2 + 3o2 = co2 + 2so2 , how many mol of o2 would react with 34.5 mol of co2?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1

Chemistry, 22.06.2019 18:30
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2

Chemistry, 22.06.2019 20:00
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1
You know the right answer?
In the balanced equation , cs2 + 3o2 = co2 + 2so2 , how many mol of o2 would react with 34.5 mol of...
Questions





Advanced Placement (AP), 26.03.2021 07:40

Computers and Technology, 26.03.2021 07:40

Computers and Technology, 26.03.2021 07:40




Mathematics, 26.03.2021 07:40

English, 26.03.2021 07:40


English, 26.03.2021 07:40

Mathematics, 26.03.2021 07:40





Mathematics, 26.03.2021 07:40

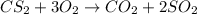
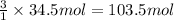 oxygen gas.
oxygen gas.

