Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
The ph of carrots are 5.0 how it is classified a.acidic b.basic c.indicator d.neutral
Answers: 2

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 08:00
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1

You know the right answer?
Given the following equation: 2h2o --> 2h2 +o2 what mass of oxygen would form from 2.30 moles o...
Questions


Biology, 22.01.2021 03:40

Mathematics, 22.01.2021 03:40




Chemistry, 22.01.2021 03:40


Mathematics, 22.01.2021 03:40


Mathematics, 22.01.2021 03:40


Chemistry, 22.01.2021 03:40

History, 22.01.2021 03:40


Business, 22.01.2021 03:40

Mathematics, 22.01.2021 03:40



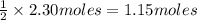
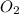 is 32 g/mol
is 32 g/mol