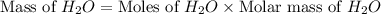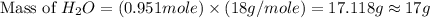Chemistry, 30.06.2019 04:30 rosehayden21
Using the following equation 2c2h6 +7o2 --> 4co2 +6h2o if 9.5g c2h6 react with 130g of o2, how many grams of water will be produced? question options: 32 41 9 17

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 11:00
When hydrochloric acid reacts with potassium hydroxide solution, the following reaction occurs. hcl (aq) + koh (aq) h2o (l) + kcl (aq) the reaction gives off heat energy, so it is an reaction.
Answers: 1

Chemistry, 22.06.2019 18:50
At stp, which substance is the best conductor of electricity? a. nitrogen b. neon c. sulfur d. silver
Answers: 1

Chemistry, 22.06.2019 20:00
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3
You know the right answer?
Using the following equation 2c2h6 +7o2 --> 4co2 +6h2o if 9.5g c2h6 react with 130g of o2, how ma...
Questions

Mathematics, 05.05.2020 14:54

Computers and Technology, 05.05.2020 14:54


History, 05.05.2020 14:54


English, 05.05.2020 14:54

History, 05.05.2020 14:54

Mathematics, 05.05.2020 14:54

Arts, 05.05.2020 14:54



Chemistry, 05.05.2020 14:54

Mathematics, 05.05.2020 14:55

Mathematics, 05.05.2020 14:55


History, 05.05.2020 14:55


Mathematics, 05.05.2020 14:55

Physics, 05.05.2020 14:55

English, 05.05.2020 14:55

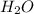 produced will be, 17 grams.
produced will be, 17 grams.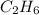 = 9.5 g
= 9.5 g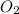 = 130 g
= 130 g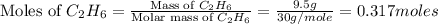
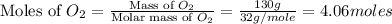
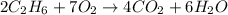
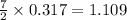 moles of
moles of 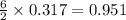 moles of
moles of