
Chemistry, 30.06.2019 03:30 gtjermaine3249
What is the relationship between atmospheric pressure and the density of gas particles in an area of increasing pressure? as air pressure in an area increases, the density of the gas particles in that area decreases. as air pressure in an area increases, the density of the gas particles in that area increases. as air pressure in an area increases, the density of the gas particles in that area remains constant. as air pressure in an area increases, the density of the gas particles in that area increases and decreases in an alternating pattern.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which type of bonding involves the complete transfer of a valence electron from a less electrogrative atom to a more electronegative one
Answers: 1

Chemistry, 22.06.2019 06:00
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1

Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1

Chemistry, 22.06.2019 13:30
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1
You know the right answer?
What is the relationship between atmospheric pressure and the density of gas particles in an area of...
Questions

Mathematics, 09.03.2021 16:40


Geography, 09.03.2021 16:40


Health, 09.03.2021 16:40

Social Studies, 09.03.2021 16:40

Biology, 09.03.2021 16:40

Mathematics, 09.03.2021 16:40

History, 09.03.2021 16:40

Biology, 09.03.2021 16:40


Mathematics, 09.03.2021 16:40



History, 09.03.2021 16:40

Mathematics, 09.03.2021 16:40

Mathematics, 09.03.2021 16:40


Computers and Technology, 09.03.2021 16:40

Social Studies, 09.03.2021 16:40

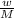 RT [where, w= mass of substance, M=molar mass of substance]
RT [where, w= mass of substance, M=molar mass of substance]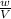 RT
RT

