
Chemistry, 30.06.2019 02:30 brooke3493
Which statement is true? a) breaking a chemical bond releases energy. b) when energy is absorbed, the reaction is said to be endothermic and â†h is negative (–). c) bond dissociation energies are always positive numbers. d) the stronger the bond, the lower its bond dissociation energy. 2. based on the reaction, n2 + o2 → 2 no â†h = 43.2 kcal which statement is true? a) 43.2 kcal are consumed when 14.00 g of n2 reacts. b) 43.2 kcal are produced when 1.00 g of o2 reacts. c) 43.2 kcal are produced when 1.00 mole of o2 reacts. d) 43.2 kcal are consumed when 2.00 moles of no is produced.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Chemical energy is a form of a. kinetic energy only. b. both potential and kinetic energy. c. neither potential nor kinetic energy. d. potential energy only. reset
Answers: 1

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3
You know the right answer?
Which statement is true? a) breaking a chemical bond releases energy. b) when energy is absorbed, t...
Questions


Mathematics, 25.02.2021 19:50


World Languages, 25.02.2021 19:50

Spanish, 25.02.2021 19:50

Mathematics, 25.02.2021 19:50



Computers and Technology, 25.02.2021 19:50





Mathematics, 25.02.2021 19:50




Mathematics, 25.02.2021 19:50


Mathematics, 25.02.2021 19:50

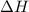 will be positive not negative.
will be positive not negative.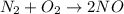
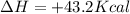
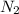 is reacted.
is reacted.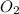 is reacted.
is reacted.

