Chemistry, 30.06.2019 00:00 TH3L0N3W0LF
Considering the limiting reactant, what is the mass of manganese produced from 25.0 g of manganese(iv) oxide (86.94 g/mol) and 25.0 g of aluminum metal? 3 mno2(l) + 4 al(l) 3 mn(l) + 2 al2o3(s)

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 04:30
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1

Chemistry, 22.06.2019 05:30
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1
You know the right answer?
Considering the limiting reactant, what is the mass of manganese produced from 25.0 g of manganese(i...
Questions

Spanish, 21.09.2020 17:01

History, 21.09.2020 17:01









Spanish, 21.09.2020 17:01

Geography, 21.09.2020 17:01

Advanced Placement (AP), 21.09.2020 17:01



Mathematics, 21.09.2020 17:01


History, 21.09.2020 17:01

Mathematics, 21.09.2020 17:01

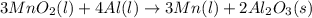
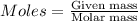 .....(1)
.....(1)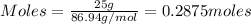
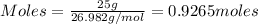
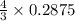 = 0.3833 moles of aluminium.
= 0.3833 moles of aluminium.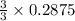 = 0.2875 moles of manganese.
= 0.2875 moles of manganese.