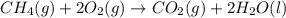Chemistry, 30.06.2019 00:00 alexwlodko
The combustion of methane gas (ch4) forms co2(g)+ h2o(ℓ). calculate the heat produced by burning 1.9 mol of the methane gas. the standard molar enthalpy of combustion of methane is 890.2kj/mol. answer in units of kj

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:10
What does a particular point on a line of a phase diagram represent? o a. the maximum temperature a substance can exist at without bonds breaking b. the pressure created by the kinetic energy of molecules at a particular temperature c. the melting point or boiling point of a substance at a specific pressure d. the conditions in which temperature and pressure have equal effects on a substance
Answers: 2

Chemistry, 22.06.2019 08:30
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2

Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1

Chemistry, 23.06.2019 00:20
How many lone pairs of electrons are on the central atom of no3- and what is the molecular shape? one, trigonal planar zero, trigonal pyramidal zero, trigonal planar one, tetrahedral one, trigonal pyramidal
Answers: 1
You know the right answer?
The combustion of methane gas (ch4) forms co2(g)+ h2o(ℓ). calculate the heat produced by burning 1.9...
Questions

Mathematics, 07.04.2020 18:02

Geography, 07.04.2020 18:02

History, 07.04.2020 18:02

Mathematics, 07.04.2020 18:02






Biology, 07.04.2020 18:02






History, 07.04.2020 18:02