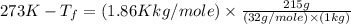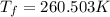Chemistry, 29.06.2019 23:30 FunnySkittle
Asolution is prepared by dissolving 215 grams of methanol, ch3oh, in 1000. grams of water. what is the freezing point of this solution? [the freezing point depression constant for water is 1.86°c/mole solute in 1000g of water]

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:40
Describe in detail the melting point behavior of the 80: 20 benzoic acid-mandelic acid mixture
Answers: 3

Chemistry, 22.06.2019 00:00
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 22.06.2019 02:00
Which of the following happens during cell division? (a) energy is created (b) waste is eliminated (c) carbon dioxide is released (d) damaged cells are replaced
Answers: 1

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1
You know the right answer?
Asolution is prepared by dissolving 215 grams of methanol, ch3oh, in 1000. grams of water. what is t...
Questions

Social Studies, 19.07.2019 20:30


History, 19.07.2019 20:30


Social Studies, 19.07.2019 20:30




Health, 19.07.2019 20:30

Health, 19.07.2019 20:30

Health, 19.07.2019 20:30

Social Studies, 19.07.2019 20:30

Chemistry, 19.07.2019 20:30







Health, 19.07.2019 20:30

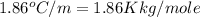
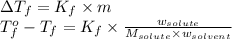
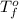 = freezing point of water =
= freezing point of water = 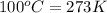
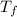 = freezing point of solution
= freezing point of solution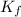 = freezing point constant
= freezing point constant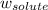 = mass of solute
= mass of solute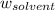 = mass of solvent
= mass of solvent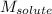 = molar mass of solute
= molar mass of solute