
Chemistry, 29.06.2019 22:30 mayamcmillan11
This chemical equation is not balanced: agbr(s) → ag(s) + br2(g). why isn’t it possible for the reaction to occur as indicated by the unbalanced equation? a. there are more substances on the right side of the equation than on the left side. b. there is a gas on the right side of the equation but not on the left side. c. the bromine atoms must go through a liquid state before becoming a gas. d. there is more mass represented on the right side of the equation than on the left side. e. the bromine atoms on the right side of the equation are not bonded to another element.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Smog is the term used to describe the combination of fog and smoke
Answers: 1

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1

Chemistry, 22.06.2019 20:00
If one fission reaction of a uranium-235 atom produced two neutrons, how many neutrons would be released if the chain reaction occurred three more times?
Answers: 1

Chemistry, 22.06.2019 21:30
If 22.5 of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. what is the new volume?
Answers: 1
You know the right answer?
This chemical equation is not balanced: agbr(s) → ag(s) + br2(g). why isn’t it possible for the rea...
Questions


Mathematics, 21.07.2020 17:01



Biology, 21.07.2020 17:01

Computers and Technology, 21.07.2020 17:01


Biology, 21.07.2020 17:01












Physics, 21.07.2020 17:01

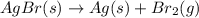 (unbalanced reaction)
(unbalanced reaction)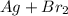 = 108 + 2(80) = 268 g
= 108 + 2(80) = 268 g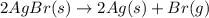 (Balanced reaction)
(Balanced reaction)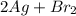 = 2(108) + 2(80) = 376 g
= 2(108) + 2(80) = 376 g

