
Chemistry, 29.06.2019 20:30 nickocasamplonp6mlob
Sucrose (c12h22o11, table sugar) is oxidized in the body by o2 via a complex set of reactions that ultimately produces co2(g) and h2o(l) and releases 5.16 ã 103 kj of heat per mole of sucrose. (a) write a balanced thermochemical equation for this reaction. include the physical state of each reactant and product. â enter your answer for î´hrxn in scientific notation. î´hrxn

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3

Chemistry, 23.06.2019 04:31
How many grams of iron can be made from 16.5 grams of fe2o3
Answers: 1
You know the right answer?
Sucrose (c12h22o11, table sugar) is oxidized in the body by o2 via a complex set of reactions that u...
Questions

English, 18.06.2020 22:57





Mathematics, 18.06.2020 22:57




Computers and Technology, 18.06.2020 22:57

Mathematics, 18.06.2020 22:57

Biology, 18.06.2020 22:57


Mathematics, 18.06.2020 22:57

English, 18.06.2020 22:57




Computers and Technology, 18.06.2020 22:57

Business, 18.06.2020 22:57

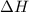 ) in a chemical reaction.
) in a chemical reaction.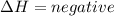 energy is released.
energy is released.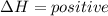 energy is absorbed.
energy is absorbed.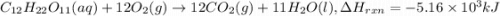
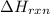 that is
that is  .
.

