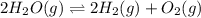Chemistry, 29.06.2019 18:30 johnsonkia873
Consider the following reversible reaction. 2h2o(g)< —> 2h2(g)+o2(g) what is the equilibrium constant expression for the given system?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1

Chemistry, 23.06.2019 06:50
The student repeated the experiment using a higher concentration of acid. the same volume of acid and the same mass of magnesium ribbon were used. what volume of hydrogen gas would have been produced after 60 seconds?
Answers: 1

Chemistry, 23.06.2019 08:00
The goal of this experiment was to answer the question "what is the effect of a gas' temperature on its volume? " you formulated the hypothesis below. hypothesis: if a fixed amount of gas is heated, then the volume will increase because the heat will cause the molecules of gas to move faster and further apart. to test this hypothesis, you changed the of the gas between 0 and 100°c (273 and 373 k) and calculated the resulting of the gas.
Answers: 2

Chemistry, 23.06.2019 11:50
Charles's law describes the relationship of the volume and temperature of gas at a constant mass and pressure. according to this law, what would happen to the temperature of the gas if its volume decreased from 1.0 l to 0.50 l?
Answers: 3
You know the right answer?
Consider the following reversible reaction. 2h2o(g)< —> 2h2(g)+o2(g) what is the equilibrium c...
Questions


Mathematics, 08.01.2021 04:00

Computers and Technology, 08.01.2021 04:00

Mathematics, 08.01.2021 04:00


Engineering, 08.01.2021 04:00

Mathematics, 08.01.2021 04:00



Physics, 08.01.2021 04:00


Mathematics, 08.01.2021 04:00

Biology, 08.01.2021 04:00


Mathematics, 08.01.2021 04:00

Mathematics, 08.01.2021 04:00

Chemistry, 08.01.2021 04:00



![k_{eq}=\frac{[H_2]^2[O_2]}{[H_2O]^2}](/tpl/images/1169/7128/9c403.png)