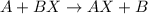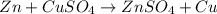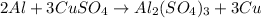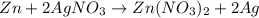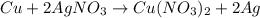Chemistry, 29.06.2019 17:30 jahnoibenjamin
1. for part 2: single-displacement reactions: for each of the four single-displacement reactions, describe what happened in each well. if a chemical reaction occurred, write a balanced equation for it. then using the a, b symbols, write a general equation for a single-displacement reaction. here are the chemical formulas of the reactants for each reaction: • zinc – zn • copper sulfate – • aluminum – al • copper sulfate – • zinc – zn • silver nitrate – • copper – cu • silver nitrate –

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3

Chemistry, 22.06.2019 23:00
Arectangle has a diagonal 20 inches long that forms angles of 60 and 30 with the sides. find the perimeter of the rectangle. for geometry
Answers: 3

Chemistry, 23.06.2019 02:00
The bohr model of the atom explained why emission spectra are discrete. it could also be used to explain the photoelectric effect. which is a correct explanation of the photoelectric effect according to the model?
Answers: 3
You know the right answer?
1. for part 2: single-displacement reactions: for each of the four single-displacement reactions,...
Questions