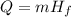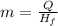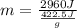Chemistry, 29.06.2019 14:00 karina1466
What is the mass of a titanium alloy that has a heat of fusion of 422.5 joules/gram and requires 2,960 joules of energy to melt completely? use the formula q = mhf. a. 0.143 g b. 1.43 g c. 7.01 g d. 7.81 g

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
14. complete and balance the equations for the single displacement reactions. a. zn + pb(no3)2 -> b. al + niso4 -> 15. complete and balance the equations for the double displacement reactions. a. agno3(aq) + nacl(aq) -> b. mg(no3)2(aq) + koh(aq) -> 16. complete and balance the equations for the combustion reactions. a. __ ch4 + o2 -> b. __ c3h6 + o2 -> c. + o2 ->
Answers: 2


Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 1

Chemistry, 22.06.2019 00:30
Which compounds have the empirical formula ch2o? a.c2h4o2 b.c3h6o3 c.ch2o2 d.c5h10o5 e.c6h12o6
Answers: 3
You know the right answer?
What is the mass of a titanium alloy that has a heat of fusion of 422.5 joules/gram and requires 2,9...
Questions

Mathematics, 13.07.2019 02:00

History, 13.07.2019 02:00

Mathematics, 13.07.2019 02:00

Mathematics, 13.07.2019 02:00

Physics, 13.07.2019 02:00


Mathematics, 13.07.2019 02:00


Mathematics, 13.07.2019 02:00


Advanced Placement (AP), 13.07.2019 02:00

English, 13.07.2019 02:00


History, 13.07.2019 02:00

English, 13.07.2019 02:00



Mathematics, 13.07.2019 02:00

English, 13.07.2019 02:00