
Chemistry, 29.06.2019 08:30 Calumworthy6046
Asealed container was filled with 0.300 mol h2(g), 0.400 mol i2 (g), and 0.200 mol hi (g) at 870k and total pressure 1.00 bar. calculate the amounts of the components in the mixture at equilibrium given that k = 870 for the reaction h2 (g) + i2 (g) ⇜ 2 hi (g).

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:20
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3

Chemistry, 22.06.2019 07:20
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1

Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3
You know the right answer?
Asealed container was filled with 0.300 mol h2(g), 0.400 mol i2 (g), and 0.200 mol hi (g) at 870k an...
Questions


Mathematics, 09.02.2021 02:00







Mathematics, 09.02.2021 02:00

Mathematics, 09.02.2021 02:00

English, 09.02.2021 02:00



English, 09.02.2021 02:00

Arts, 09.02.2021 02:00

Mathematics, 09.02.2021 02:00

Business, 09.02.2021 02:00

Biology, 09.02.2021 02:00

Mathematics, 09.02.2021 02:00

History, 09.02.2021 02:00

![[HI] _{eq}=0.825mol](/tpl/images/0030/3484/a93b2.png)
![[H_2] _{eq}=0.010mol](/tpl/images/0030/3484/29662.png)
![[I_2] _{eq}=0.078mol](/tpl/images/0030/3484/3a542.png)
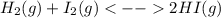
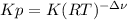 ):
):![\frac{Kp}{(RT)^{2-2} }=\frac{[HI]^{2}_{eq} }{[H_2]_{eq}[I_2]_{eq}} \\Kp=\frac{[HI]^{2}_{eq} }{[H_2]_{eq}[I_2]_{eq}}](/tpl/images/0030/3484/98372.png)
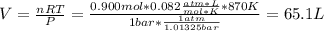
![[H_2] _0=\frac{0.300mol}{65.1L}=0.005M](/tpl/images/0030/3484/0ef57.png)
![[I_2] _0=\frac{0.400mol}{65.1L}=0.006M](/tpl/images/0030/3484/d9a8e.png)
![[HI] _0=\frac{0.200mol}{65.1L}=0.003M](/tpl/images/0030/3484/e3fd2.png)
 due to the equilibrium:
due to the equilibrium: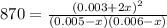
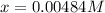
![[HI] _{eq} =(0.003M +2(0.00484M))*65.1L=0.825mol](/tpl/images/0030/3484/1d681.png)
![[H_2] _{eq} =(0.005M -0.00484M)*65.1L=0.010mol](/tpl/images/0030/3484/34688.png)
![[I_2] _{eq} =(0.006M -0.00484M)*65.1L=0.078mol](/tpl/images/0030/3484/28b57.png)


