
Chemistry, 29.06.2019 05:00 natishtaylor1p8dirz
An atom of sodium-23 (na-23) has a net charge of +1. identify the number of protons, neutrons, and electrons in the atom. then, explain how you determined the number of each type of particle.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
You are performing an experiment in a lab to attempt a new method of producing pure elements from compounds. the only problem is that you do not know what element will form. by your previous calculations you know that you will have 6.3 moles of product. when it is complete, you weigh it and determine you have 604.4 grams. what element have you produced?
Answers: 1

Chemistry, 21.06.2019 21:30
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 21.06.2019 21:30
Sex cells from female reproductive organ? 1) mitosis 2) fertilization 3) zygote 4) eggs 5) meiosis 6) sperm
Answers: 2

Chemistry, 21.06.2019 22:30
Embryos of different species look very similar, which shows that the organisms share a ancestor.
Answers: 1
You know the right answer?
An atom of sodium-23 (na-23) has a net charge of +1. identify the number of protons, neutrons, and e...
Questions

Social Studies, 08.12.2019 18:31



Mathematics, 08.12.2019 18:31


Mathematics, 08.12.2019 18:31




History, 08.12.2019 18:31

Mathematics, 08.12.2019 18:31

History, 08.12.2019 18:31


History, 08.12.2019 18:31

Social Studies, 08.12.2019 18:31


Computers and Technology, 08.12.2019 18:31


History, 08.12.2019 18:31

Mathematics, 08.12.2019 18:31

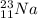
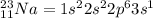
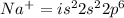 (It will loose one electron)
(It will loose one electron)

