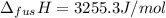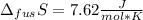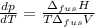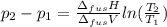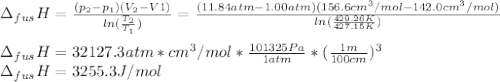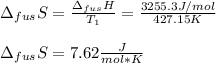The molar volume of a certain solid is 142.0 cm3 mol−1 at 1.00 atm and 427.15 k, its melting temperature. the molar volume of the liquid at this temperature and pressure is 152.6 cm3 mol−1. at 1.2 mpa the melting temperature changes to 429.26 k. calculate the enthalpy and entropy of fusion of the solid

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Plz choose one of the compounds from the table and explain how you know the numbers of atoms in your formula. is it possible for two different compounds to be made from the exact same two elements? why or why not? with a limited number of elements (less than 120 are known), does this mean we also have a small number of compounds or do we have a large number of compounds in this world?
Answers: 1

Chemistry, 22.06.2019 00:30
The clouds are grey and ground is wet. a quantitative b qualitative
Answers: 1

Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 07:00
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2
You know the right answer?
The molar volume of a certain solid is 142.0 cm3 mol−1 at 1.00 atm and 427.15 k, its melting tempera...
Questions


English, 27.04.2021 22:00




Physics, 27.04.2021 22:00

Mathematics, 27.04.2021 22:00

Mathematics, 27.04.2021 22:00


Mathematics, 27.04.2021 22:00


Chemistry, 27.04.2021 22:00



Advanced Placement (AP), 27.04.2021 22:00