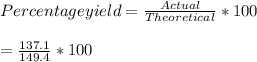Chemistry, 29.06.2019 02:00 101EXPERIENCE
Consider the following reaction: 2h2s (g) + 3o2 (g) 2so2 (g) + 2h2o (g) if o2 was the excess reagent, 8.3 mol of h2s were consumed, and 137.1 g of water were collected after the reaction has gone to completion, what is the percent yield of the reaction? show your work.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:40
You may expect bonds between two atoms which each have n covalent lonic metallic hydrogen
Answers: 2

Chemistry, 22.06.2019 05:30
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3


You know the right answer?
Consider the following reaction: 2h2s (g) + 3o2 (g) 2so2 (g) + 2h2o (g) if o2 was the excess reag...
Questions




Mathematics, 23.07.2019 20:00




Mathematics, 23.07.2019 20:00





SAT, 23.07.2019 20:00